We know that a common substance like water is found naturally as a solid, liquid otherwise gas, and these are called states of matter. In solids, the arrangement of molecules can be done very close to each other because the molecules in the atoms move into the orbitals of adjacent atoms. In liquids, the arrangement of molecules is very close to each other whereas, in gases, the arrangement of molecules can be spread apart, so not very close to each other. Therefore, once atoms come together, then the electron orbitals will overlap. In solids, different energy bands can be formed because of the blending of atoms, so we can call the set of energy levels like energy bands. This article discusses an overview of energy band and their types
What is Energy Band?
Energy band definition is, according to Bohr’s theory, every shell and associate shell of atoms include a discrete quantity of energy. An atom contains different energy levels and once atoms are brought nearer to each other, then electrons at the outside shell will interact with each other. So, this bonding force among electrons is known as inter-atomic communication.
This communication can cause the change within energy levels of electrons at the outside shell which will increase energy band theory. Thus, the range of electrons will not be at a similar range, the electrons ranges will be changed to a value that is lower or higher as compared to the original level. Every substance includes the different quantity of electron energy that is present within the energy bands depending on these energy levels.
Energy Band Theory
Based on Bohr’s theory, each shell in an atom includes a separate quantity of energy on different levels. So, energy band theory states that the communication of electrons among the external and internal shells. There are three different energy bands based on the energy bands theory: Energy bands are classified into three types like following.
- Valence band
- Forbidden Energy Gap
- Conduction Band
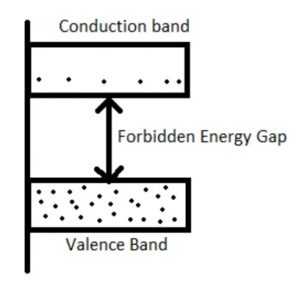
Energy Band Theory
Valence Band
In solids, different ranges of energies can be present at complete zero temperature and the band which can be formed through the maximum energy range is known as the valence band which includes valence electrons.
To form a solid, atoms are bringing nearer together, the levels of discrete energy are interrupted due to the quantum mechanical effects. In an individual atom group, the electrons can occupy a band of levels within the solids which is called a valence band. The formation of this band can be done through the electrons at an outside shell.
In this band, the electrons have less energy as compared to the electrons within the conduction band. In atoms, the valence band electrons freely jump to the nucleus. In solids, the electrical conductivity mainly depends on the capacity to flow the electrons from one band to other like valence to conduction.
Forbidden Energy Level
This energy level is also called the Fermi energy level. In this energy band, the electron state will exist because of quantization energy. The band which is obtained through dividing two bands like conduction & valence is known as the forbidden gap of the forbidden energy band.
In electrons in solids do not wait in the forbidden gap due to the absence of an energy state within the region. The solid’s electrical conductivity can be determined by using a forbidden gap.
Conduction Band
The conduction band can be defined as the energy band which can be formed through the levels of free electrons energy. This band is a partially filled band or an empty band; however, once the exterior field is given to the electrons within the valence band, then the electrons will jump from one band to other like the valence to the conduction & turns into a free electron.
As compared to the valence band, the electrons within the conduction band have maximum energy. In this band, electrons cannot jump into the center of the atom. This band can be arranged on the Fermi level.
The diagram for the valance & conduction bands and the forbidden gap is shown below. Here, based on the forbidden gap size, formation of semiconductors, insulators & conductors can be formed. There are three types of energy bands like the following.
Types of Energy Bands
In energy bands, the bandgap can be defined as the small amount of energy necessary for a flow of electrons to break free of its bounce state. Once the energy of the bandgap is met, then the electron can be excited into a free condition, thus it can participate within conduction.
The bandgap will decide how much energy can be required from the sun for transmission and also how much energy can be generated. The formation of the hole can be done where the electron jumps and this hole can also take part within conduction.
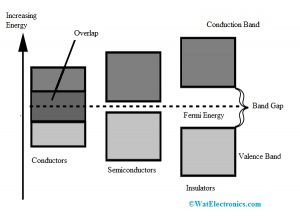
Energy Band
Conductors
Conductors are metals that allow the flow of current like aluminum, copper, gold, silver, etc. In conductors, there is no forbidden gap between the two bands like valence and conduction which is shown in the energy band diagram. So these two bands will overlap. At room temperature, the flow of electrons which are available will be high.
Properties
The properties of conductors include the following.
- In conductors, there is no forbidden gap between two bands
- The two bands will get overlapped.
- For conduction, fewer amounts of electrons will be available.
- Conduction will be increase when voltage increases slightly.
- There is no formation of the hole because a continuous electrons flow will supply the current.
- A conductor allows the flow of ions and electrons through them.
- Free charges will exit simply on the outside of the conductor
- The conductor’s electric field can be zero permitting the flow of electrons in them.
- The conductor’s charge density is zero.
- In a conductor, there is no forbidden gap
- The two bands within the conductor can be overlapped
- For conduction, fewer electrons will be available
- The conduction will be increased once the voltage increases slightly
Insulators
Insulators do not allow the flow of current and the best examples are wood, glass, etc. Insulators have less conductivity and high sensitivity. The energy bandgap within the insulator is extremely high like 7eV. These materials cannot conduct because there is no flow of electrons from one band to other like valence to the conduction. The energy bandgap diagram of insulators is shown below.
Properties
The properties of insulators include the following.
- In insulators, the forbidden energy gap is extremely low.
- The electrons within the valance band can jump strongly to atoms.
- For insulators, the forbidden energy gap is 10eV.
- When the temperature increases in some insulators, then there is some conduction.
- It includes large resistance & specific resistance.
- It has high dielectric strength.
- Mechanical strength is high
Semiconductors
The electrical properties of materials that lie among the semiconductors & insulators are known as semiconductors. Examples of semiconductors are Silicon and Germanium. The energy bandgap diagram of semiconductors is shown below. The forbidden gap among the valence and conduction bands in semiconductors is less. The energy bandgap of semiconductor, silicon, germanium is; for Semiconductor less than 1 eV, for Silicon-1.1eV, and for Germanium it is 0.72eV.
Properties
The properties of semiconductors include the following.
- In semiconductors, the forbidden energy gap is extremely less.
- A Semiconductor is neither a good conductor nor an insulator
- The semiconductor’s conductivity will be increased when the temperature increases.
- The semiconductor’s conductivity will be 102 mho-meter.
Thus, this is all about an overview of an energy band gap, theory, and its different types with properties. Energy band theory is nothing but the interaction between the innermost and outermost shells. The classification of energy bands can be done based on the theory of energy bands like valence bands, forbidden bands, and conduction bands. Here is a question for you, what is the energy gap?